Thermodynamic Analysis of Naphthyl Compounds by Pyrolysis to Hydrogen
DOI:
https://doi.org/10.37934/sijpce.3.1.6881Keywords:
FOBS, pyrolysis, naphthyl compounds, hydrogenAbstract
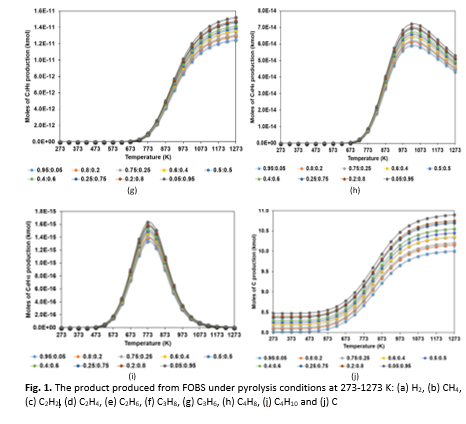
Hydrogen (H2) is a clean and versatile energy carrier with wide-ranging applications across various technologies and industries. It can be produced through several pathways, including fossil fuels, renewable resources, nuclear energy, and chemical processes. In line with efforts to support sustainable development and effective waste management, this study focuses on the production of hydrogen through pyrolysis of refinery waste, specifically fuel oil blended stock (FOBS). A thermodynamic assessment was carried out to evaluate the feasibility of hydrogen generation from this waste material. Naphthalene and 2-methylnaphthalene two dominant polycyclic aromatic hydrocarbons (PAHs) found in FOBS, were selected as representative compounds of FOBS for the modelling process. Using HSC Chemistry 6.0 software, the study applied the Gibbs free energy minimization method to simulate pyrolysis reactions across a temperature range of 273-1273 K at atmospheric pressure (1 bar). Various molar feed ratios of naphthalene to 2-methylnaphthalene were tested: 0.95:0.05, 0.8:0.2, 0.75:0.25, 0.6:0.4, 0.5:0.5, 0.4:0.6, 0.25:0.75, 0.2:0.8, and 0.05:0.95. The results indicated that hydrogen production was negligible below 500 K, but increased sharply between 673-1273 K, with the highest yield observed at higher 2-methylnaphthalene content, attributed to enhanced side-chain reactivity and dealkylation behaviour. In contrast to initial assumptions, 2-methylnaphthalene contributed more significantly to hydrogen formation than naphthalene, which primarily acted as a structural backbone during pyrolysis. These findings demonstrate the potential of FOBS as an effective feedstock for hydrogen production and at the same time parallel to a sustainable approach for clean energy generation and industrial waste reduction.