Thermodynamic Analysis of 2-Methylnaphthalene Steam Reforming to Hydrogen
DOI:
https://doi.org/10.37934/sijpce.3.1.114Keywords:
FOBS, steam reforming, 2-methylnaphthalene, hydrogen productionAbstract
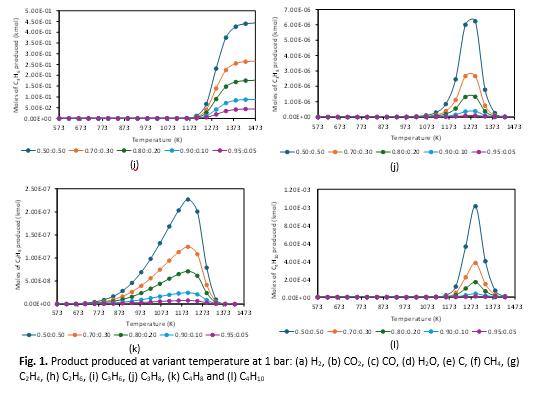
Fuel Oil Blended Stock (FOBS) is a byproduct of petroleum refining, that contains high levels of hazardous hydrocarbons, heavy metals, and sulfur compounds which present significant environmental and storage challenges. Its rich hydrocarbon content offers potential waste-to-energy that is marketable as energy sources. This study evaluates the thermodynamic feasibility of transforming FOBS using 2-methylnaphthalene as a model compound to hydrogen (H2), employing Gibbs free energy minimization in HSC Chemistry 6.0. Key thermodynamic parameters such as temperature (300–1200°C), pressure (1–100 bar), and 2-methylnaphthalene-to-water feed ratios (0.5:0.5; 0.7:0.3; 0.8:0.2; 0.9:0.1; 0.95:0.05) were investigated to determine optimal reaction conditions. Results show that a 0.50:0.50 molar ratio of 2-methylnaphthalene to water maximizes the production of hydrogen (H2), methane (CH4), carbon monoxide (CO), carbon dioxide (CO2), and other hydrocarbons (C1-C4). H2 yield peaks at 29.71% at a high temperature of 1248 K at temperature 1 bar, while excessively high temperatures lead to carbon formation, underscoring the need for precise thermal control. This thermodynamic study theoretically confirmed the feasibility of converting FOBS into sustainable fuels, contributing to energy efficiency. However, further consideration of environmental sustainability is necessary, as hydrocarbon (C1-C4) gases are also produced alongside H2.