Hydroxyl Radicals Production in Nitrogen Fixation with Addition of Fe2+ Ions using Plasma Electrolysis
DOI:
https://doi.org/10.37934/sijpce.1.1.5162aKeywords:
Hydroxyl radicals, nitrate, nitrogen fixation, plasma electrolysisAbstract
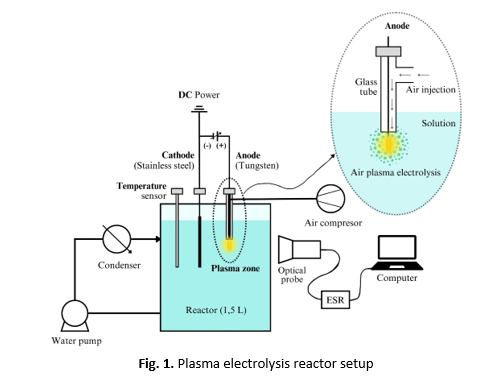
Nitrogen fixation using the Haber-Bosch process generates carbon dioxide emissions, so environmentally friendly alternative technologies are needed. Plasma electrolysis is recommended as a nitrogen fixation method that produces liquid nitrate because it does not generate emissions with raw materials from the natural air. The plasma electrolysis process generates hydroxyl radicals in the nitrate formation process. Thus, the aim of this research is to increase hydroxyl radicals by adding Fe2+ ions. The methods used include characterizing the current and voltage strength in the reactor to observe the glow discharge position for plasma formation. The analysis of hydroxyl radicals formed with the addition of Fe2+ ions was conducted using Electron Spin Resonance (ESR). The analysis of the yield of liquid nitrate formed was performed using a UV VIS Spectrophotometer. The results obtained were the emission spectrum of radicals formed in plasma without air injection. The wavelengths of the detected radicals are as follows: •OH (hydroxyl radical) at 306 nm, with an intensity of 21.696 a.u, •H (hydrogen radical) at 654 nm, with an intensity of 12.572 a.u. and •O (oxygen radical) at 844 nm, with an intensity of 13.267 a.u. When air injection is used, 0.8 liters per minute (lpm), there is a significant increase in the intensity of reactive species required for nitrate formation, such as excited nitrogen (N₂*) at 28540 a.u, hydroxyl radicals (•OH) at 30863 a.u, and oxygen (•O) at 49800 a.u. the concentration of nitrate produced reaches 1889 ppm. This is different from the condition without air injection (0 lpm), where nitrate is not formed.