Efficient Biodiesel Synthesis using Carbon-Immobilized Enzyme in a Stationary Bed Reactor
DOI:
https://doi.org/10.37934/sijcpe.4.1.6071Keywords:
Biodiesel, enzyme, immobilization, lipase, stationary bed reactorAbstract
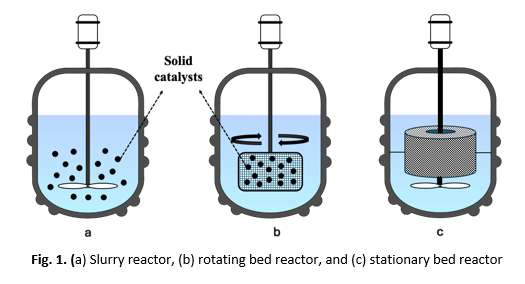
The use of catalysts in chemical reactions aims to increase the reaction rate, improving the efficiency of a chemical process. Heterogeneous catalysts from solid materials such as metals or non-metals supported by carbon have been widely developed with high activity and selectivity for various chemical reactions, including esterification and transesterification. The selection of an appropriate reactor type is crucial to enhancing the effectiveness of the catalytic reaction system. In this study, a stationary bed reactor (SBR) and a slurry reactor were compared their effectiveness for the catalytic systems. The reference reaction was the transesterification reaction between oil and methanol, catalyzed by lipase enzyme immobilized on carbon for biodiesel synthesis. The reaction was carried out at a constant temperature of 70°C for 1-4 hours with variations in the ratio of methanol to oil of 1:2 - 1:10. The result shows that the performance of the two reactors is relatively comparable. When using a stationary bed reactor (SBR), it produces a greater biodiesel yield at a higher ratio of methanol to oil. This shows that the SBR reactor effectively improves catalyst performance. Biodiesel obtained from the transesterification reaction has a density of 820-840 kg·m-3, a viscosity of 0.5-0.79 mm2·s-1, and a calorific value of ± 5000 Cal/g. From the GC-MS analysis, it is known that the composition of biodiesel consists of 21.47% methyl palmitate, 21.03% oleic acid, and 18.89% methyl oleate. The stability test showed that the catalyst was stable for up to three reaction cycles.