Thermodynamic Analysis of Glycerol Dry Reforming to Hydrogen at Low Pressure
DOI:
https://doi.org/10.37934/sijcpe.3.1.2236Keywords:
Thermodynamic modelling, Glycerol, Dry reforming, HSC Chemistry, Hydrogen productionAbstract
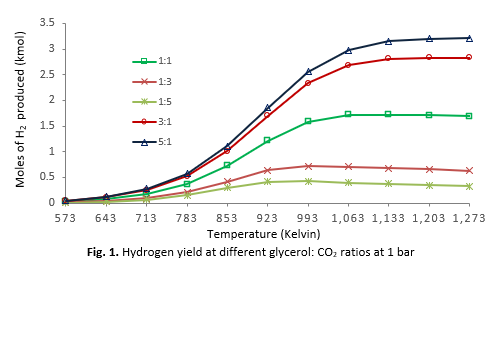
The chemical industry heavily relies on hydrogen, with approximately 95% of its production derived from fossil fuels like methane (CH4). However, dry reforming technologies face challenges related to raw material quality, conversion efficiency, and safety when integrating systems for hydrogen production. Glycerol, a by-product of biodiesel production, offers a promising alternative raw material for hydrogen generation. This study explores glycerol dry reforming method, evaluating the thermodynamic aspects using the HSC Chemistry program. The effects of reaction conditions on glycerol conversion to hydrogen (H2) and carbon monoxide (CO) were assessed through total Gibbs free energy minimization, focusing on temperature (300°C–1000°C), pressure (0.001–1.0 bar), and glycerol to CO2 ratios (1:1, 1:3, 1:5, 3:1, and 5:1). Results showed that hydrogen was the dominant product, with optimal conditions for hydrogen production at 783 K, 1 bar, and a glycerol to CO2 ratio of 5:1. Under reduced pressure (0.001 bar) and lower temperature (573 K), increasing glycerol concentration improved hydrogen yields. The findings suggest that glycerol's inclusion in the dry reforming process under low-pressure conditions enhances process efficiency and cost-effectiveness.