Critical Failure Factors for Factory Automation Project in Medical Device Assembly
DOI:
https://doi.org/10.37934/sijaset.5.1.3654Keywords:
Critical failure factors, factory automation, project management, medical device assemblyAbstract
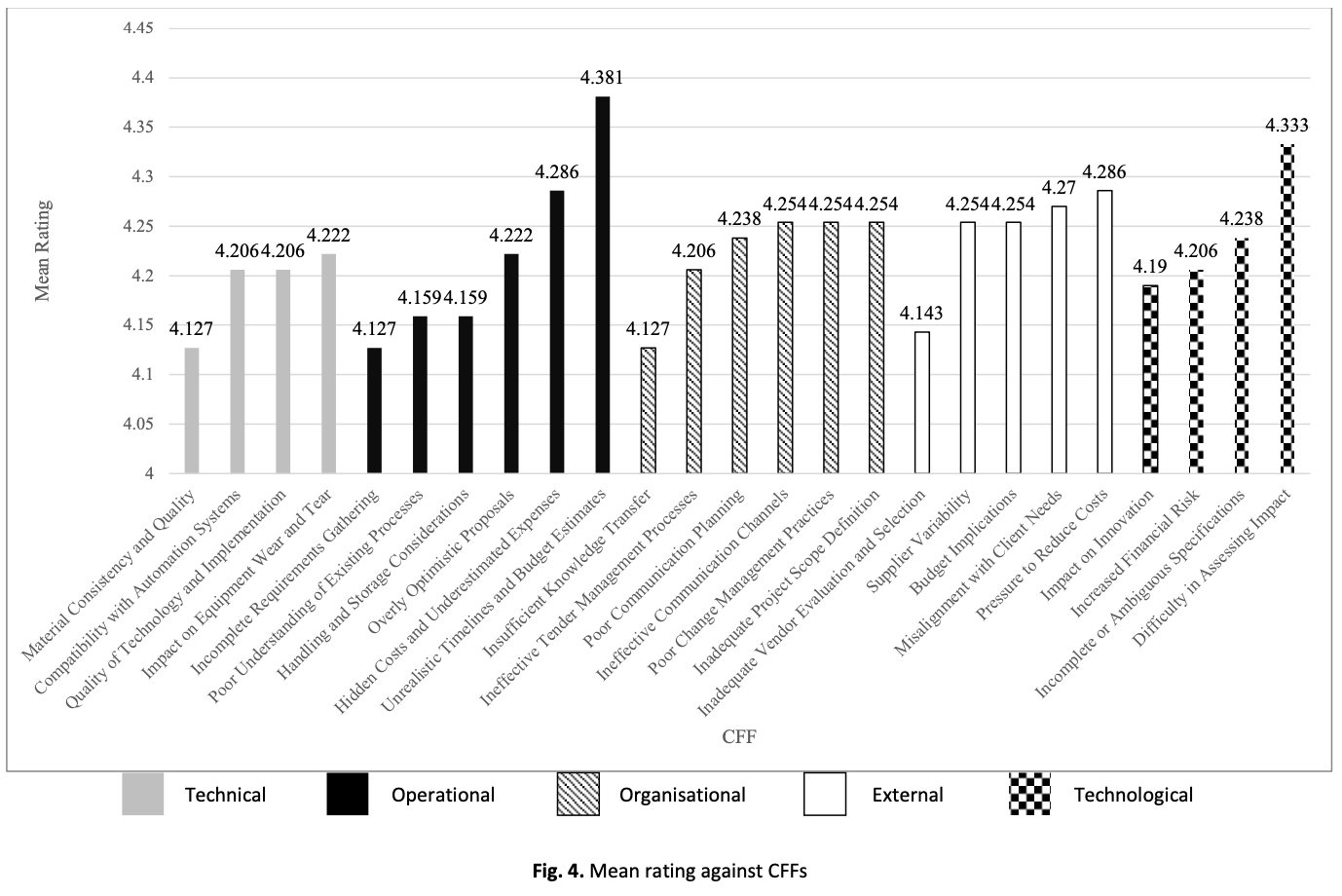
Factory automation has become essential in the medical device assembly sector to enhance efficiency, reduce costs, and maintain stringent quality standards. However, many automation projects face significant challenges, leading to delays, budget overruns, and failures. The problem stems from various factors, including technical limitations, operational inefficiencies, organisational barriers, external pressures, and technological constraints. Despite the growing adoption of automation, comprehensive studies do not categorise these failure factors and analyse their impact collectively. This study aims to identify and categorise critical failure factors (CFFs) in factory automation projects within the medical device assembly sector. A literature review was conducted to establish key failure factors, followed by a survey of 63 industry professionals. The collected data were analysed using descriptive statistics to determine the most significant failure factors affecting project success. The findings reveal that unrealistic timelines and budget estimates (operational), difficulty in assessing impact (technological), and cost reduction pressures (external) are among the most prominent challenges. The discussion highlights how these factors contribute to project failures, emphasising the interconnected nature of technical, organisational, and external influences. This study contributes to the field by offering a structured categorisation of CFFs, providing valuable insights for industry professionals to enhance project planning and execution. Future research should explore mitigation strategies and assess their effectiveness in addressing these failure factors.